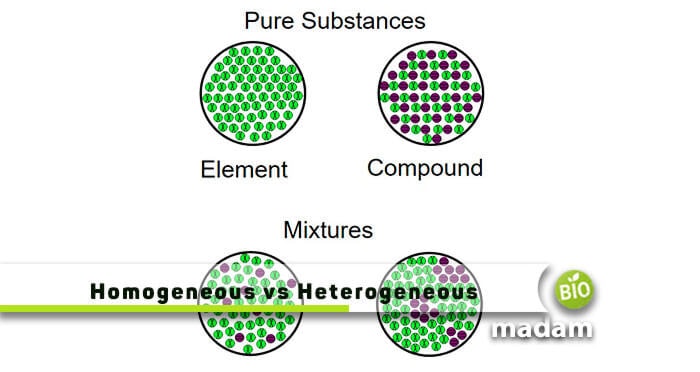
Definition (2) A homogeneous team consists of people, who are very much similar, having similar points of view, learning life experiences, and abilities A heterogeneous team includes a mixture of cultures, ages, genders, and races that gives a wider range of opinions and life experiences A homogeneous team sometimes provides equal access andAs a homogeneous mixture has two or more distinct phases By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase

Is Salty Water Homogeneous Or Heterogeneous
Heterogeneous and homogeneous mixture definition
Heterogeneous and homogeneous mixture definition- The terms heterogeneous and homogeneous refer to mixtures of materials in chemistry The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their compositionDifference between homogeneous HealthThe and a simple heterogeneous way of wine Trail To explain the differences between homogeneous and heterogeneous is to think of two substances that mix and no longer be separated, against two mixing substances that can be separated For example, if you mix salt in a glass of water and dissolve The salt,



3
Types of Mixtures A mixture is a combination of two or more substances mixing together without any chemical reaction as a result, their molecular structure does not change being not involving any chemical reaction, the substances can be separated easily at any time there are two Types of Mixtures ie, Homogeneous Mixtures and Heterogeneous mixtures Homogeneous mixtures are uniform, that is, their composition is the same wherever you look at it;Define homogeneous and heterogeneous mixture Answers 2 Get
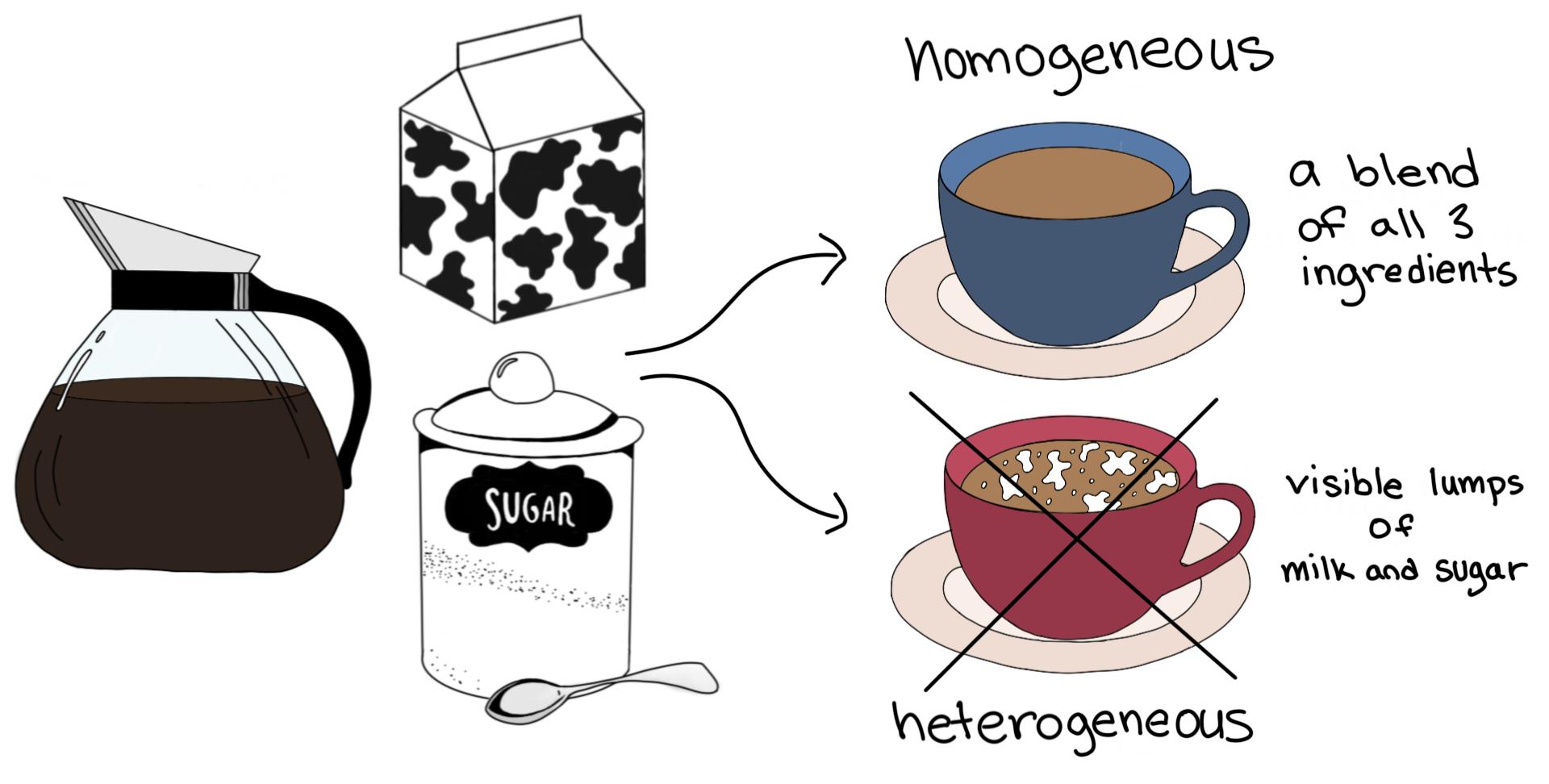
A mixture of two immiscible liquids is also a heterogeneous mixture, like a mixture of water and oil is also a heterogeneous mixture because when they both will be mixed together, the oil will start floating on the top surface, and they will not get mixed with each other forming two clear phases in the mixtureAn example of the latter would be a mixture of water, octane, and silicone greaseHeterogeneous solids, liquids, and gases may be made homogeneous by melting, stirring, or by allowing time to pass for diffusion to distribute By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers In light of this, what is the difference between a homogeneous mixture and a heterogeneous mixture quizlet?
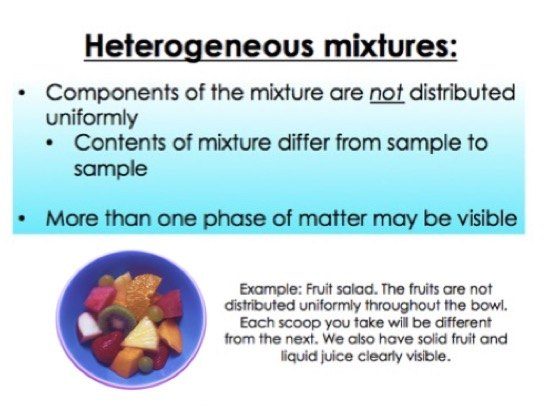
Heterogeneous mixtures A heterogeneous mixture is the one in which the substances are not distributed uniformly and the composition is also not uniform which means you can easily differentiate between the substances Properties of heterogeneous mixtures It does not have a uniform appearance The particles in the mixture are easily visibleThis video is in simple language about Difference between homogenous and heterogeneous mixturesClass 9Chapter 2Is Matter Around Us PureIn this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference between




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Mixture Examples Found At Home
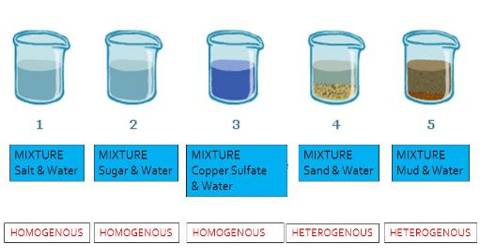
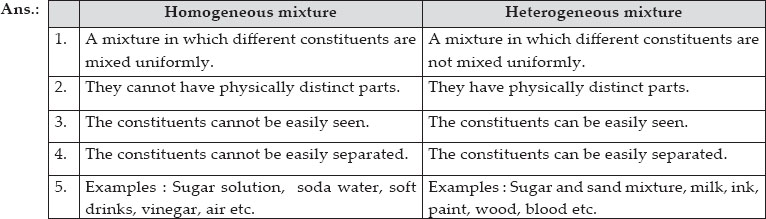
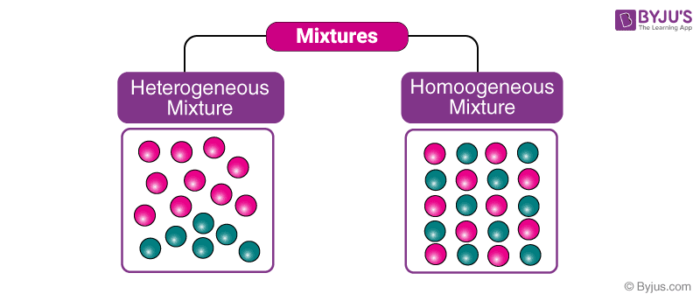
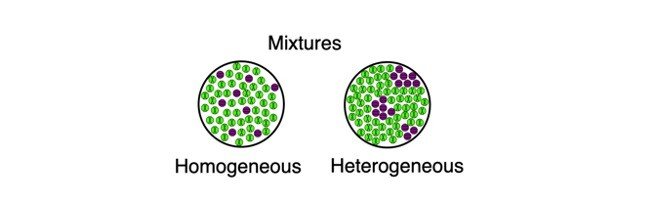
Mixtures which do not have uniform composition throughout are called Heterogeneous Mixture For example – mixture of soil and sand, mixture of sulphur and iron fillings, mixture of oil and water etc The boundaries of constituent particles of a homogeneous mixture can be identified easily;While heterogeneous mixtures are uneven, with a composition that varies from one point to another In homogeneous mixtures, there seems to be only one component (solute and solvent), but in heterogeneous, we easily visualize more than two componentsHomogeneous mixture Heterogeneous mixture 1) These are called as solutions These are called as suspensions/colloids 2) Substances are Uniformly distributed These substances are Unevenly distributed 3) These are not visible to the naked eye, but visible through the microscope
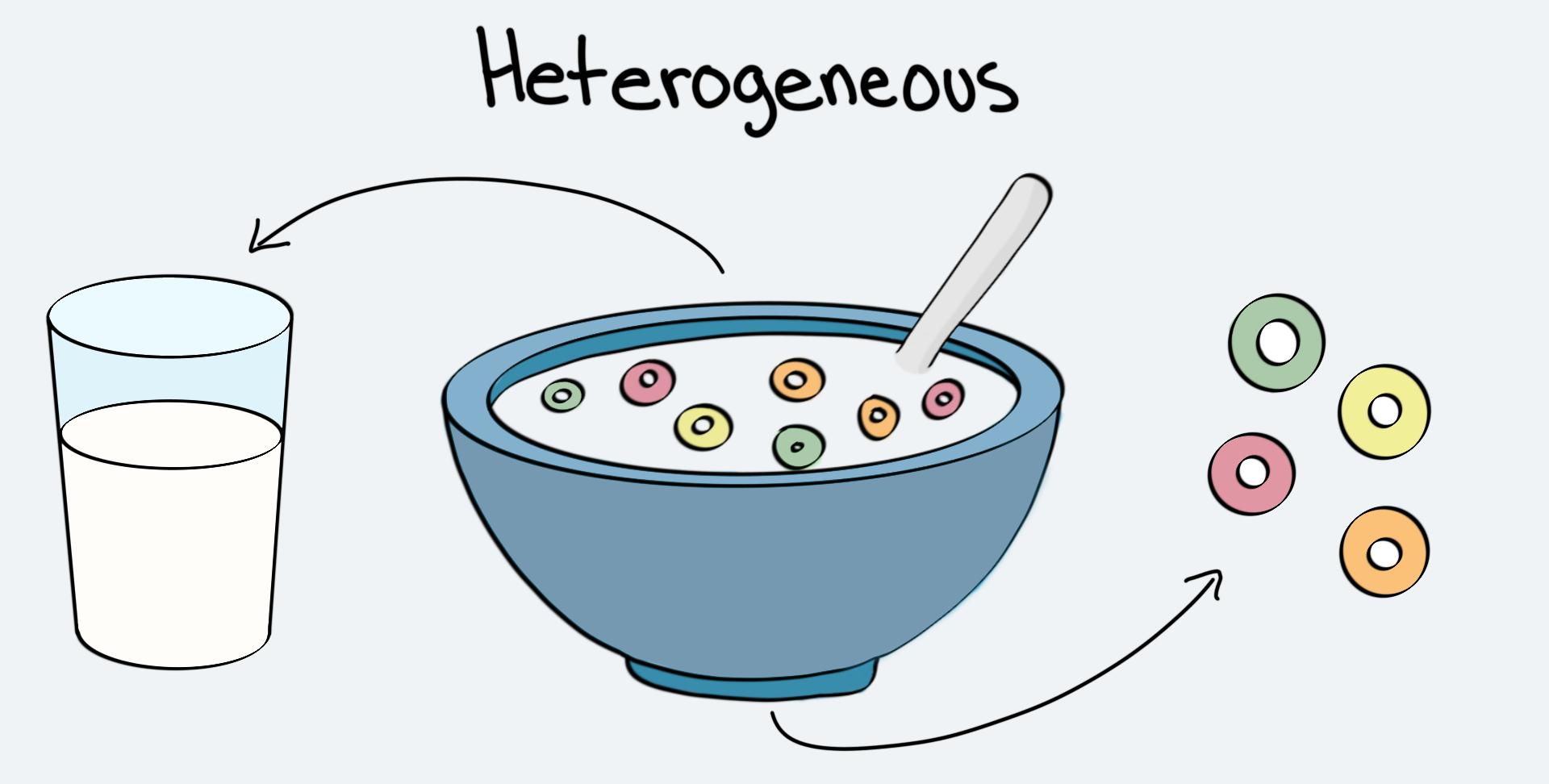



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Heterogeneous And Homogeneous Mixtures What S The Difference Homogeneous Mixture Heterogeneous Mixture Chemistry
So, a heterogeneous mixture is a substance that can be easily separated into its parts, and those parts retain their original properties A heterogeneous mixture is not blended together or the sameHomogeneous Mixture Definition A homogeneous mixture is a mixture of substances blended so thoroughly that you cannot see individual substances Every sample of the mixture will show the same amounts of each substance Homogeneous mixtures can be solid, liquid, gas, or plasma mixtures Examples include chocolate chip cookies, soda with ice, a sandwich, pizza, and tossed salad A heterogeneous mixture is defined as a mixture that has a nonuniform composition In other words, its composition varies from one location to another In contrast, a homogeneous mixture has a uniform composition Its appearance and composition are the



Is Sugar A Homogeneous Or Heterogeneous Mixture Chemistry For Neet




Homogeneous Mixture And Heterogeneous Mixture Is Matter Around Us Pure Chemistry Class 9 Youtube
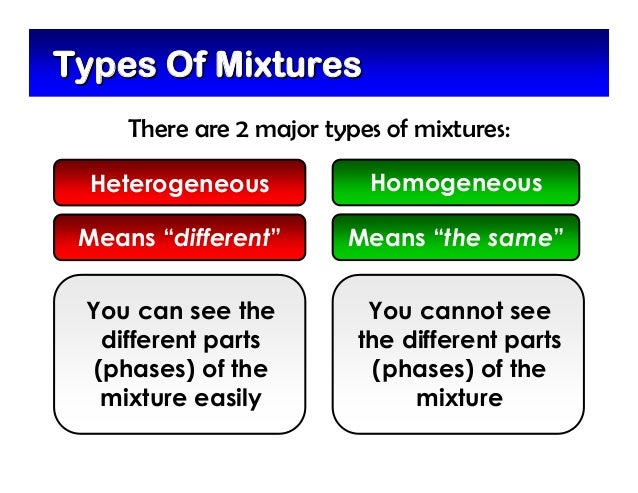
Heterogeneous and Homogeneous Definition There is some confusion over the words 'homogeneous' and 'heterogeneous' The differences between the two words, when it comes to practical, realworld applications, are quite similar but with a few key differencesHomogeneous mixtures have uniform composition Heterogeneous mixtures have nonuniform composition Example Mixture of Salt and Iron Filings Note Heterogeneous and homogeneous mixture may be a Matter of Scale It means that a mixture may look homogeneous from a distance But if we look closely with help of a microscope, it may appear to be heterogeneous mixture (We may be able to identify individual component clearly)




2 3 Pt A Define A Homogeneous Mixture And Give Chegg Com




3 4 Classifying Matter According To Its Composition Chemistry Libretexts
Define homogeneous and heterogeneous mixture???A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquids and solids Graphics to the left of "Ball Tovains" shows liquid, solid and gas substances in a heterogeneous mixture The dimensions of the particles distinguish homogeneous solutions from other heterogeneous mixturesHeterogeneous mixtures possess different properties and compositions in various parts ie the properties are not uniform throughout the mixture Examples of Heterogeneous mixtures – air, oil, and water, etc 2 What is a Homogeneous Mixture?
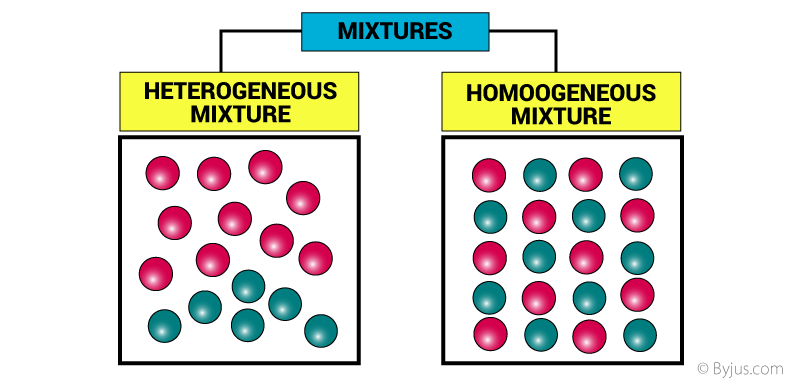



What Is A Mixture Definition Properties Examples Types With Videos



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
Correct answers 1 question Define heterogeneous and homogeneous Describe sedimentation and decantation What kind of mixtures can be separate byWhat is meant by a pair of miscible liquids ?Mixtures can be characterized by being separable by mechanical means eg heat, filtration, gravitational sorting, centrifugation etc Mixtures can be either homogeneous or heterogeneous' a mixture in which constituents are distributed uniformly is called homogeneous, such as salt in water, otherwise it is called heterogeneous, such as sand in waterHeterogeneous mixture the substances are not distributed evenly (chocolate cups, pizzas, rocks) Within the categories of homogeneous and heterogeneous mixtures there are more specific types of mixtures including solutions, alloys, suspensions and colloids
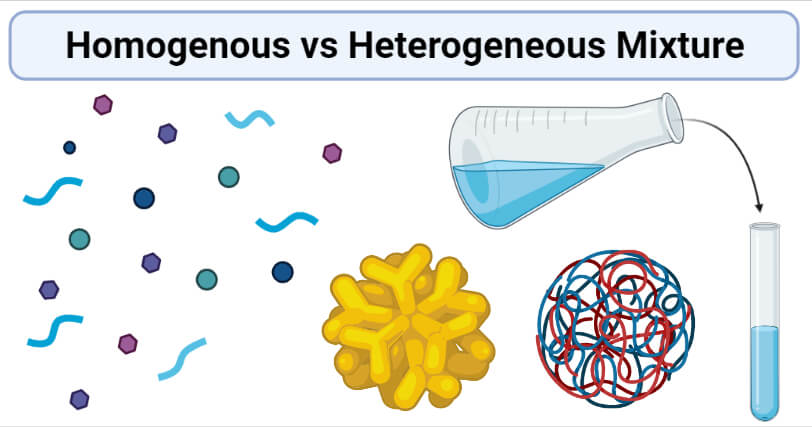



Homogenous Vs Heterogeneous Mixture Definition 8 Key Differences Examples




Difference Between Homogenous And Heterogenous Mixtures Youtube
Definition Homogeneous Mixture The mixing of two or more substances that results in uniform properties is called a homogeneous mixture Heterogeneous Mixture The mixing of two or more than two substances that do not result in uniform properties is called a heterogeneous mixture One can see the different particles added in a heterogeneousA homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample Conversely, a heteroge jangaiahgoudch1446 jangaiahgoudch1446 Chemistry Secondary School answered Definition of homogeneous and heterogeneous mixture 2 See answersSugar mixed with water is the most common example of a homogeneous mixture



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Heterogeneous Mixture Definition Science Trends
Homogeneous mixtures are also called as solutions Uniform composition Example rainwater, vinegar etc Heterogeneous mixture This is a type of mixture in which all the components are completely mixed and all the particles can be seen under a microscope We can easily identify the components and more than one phase can be seen by naked eyes HOMOGENEOUS MIXTURE Definition of the homogeneous mixture– Homogeneous mixture is the one, in which the components are uniformly distributed throughout its volume and cannot be seen separately We should not confuse a homogenous mixture with a compound In compounds atoms of various elements combine chemically in a fixed ratio Whereas, in the homogeneous mixtures, components of the mixtureIn chemistry, a heterogeneous mixture consists of either or both of a) multiple states of matter or b) hydrophilic and hydrophobic substances in one mixture;



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
Give one example of such a pairflowsheet diagram describing separation of the constituents of a mixture containing sand Homogeneous mixtures have a uniform composition throughout the system, and heterogeneous mixtures are the opposite The particles in a heterogeneous are randomly arranged whereas particles in a homogeneous mixture are arranged in a much orderly way, giving rise to a uniform composition A heterogeneous mixture is the type of mixture in which the composition of the solute is not uniform throughout the mixture Thus, in a heterogeneous mixture, all parts of the mixture do not have the same concentration throughout




Difference Between Homogeneous And Heterogeneous Material Youtube



Difference Between Homogenous And Heterogeneous Mixture Javatpoint
Homogeneous glossary a mixture in which the composition is uniform throughout the mixture Mixture it consists of more substances put together Substance it has a uniform and defined composition Learning objectives define the heterogeneous mixture Define the phaseHeterogeneous mixtures are defined as the mixture where components are mixed nonuniformly Irrespective of homogeneous mixture where components are in single phase, in heterogeneous mixture components are present in at least two different phases Properties of Heterogeneous Mixtures Heterogeneous mixture shows the following propertiesExamples of homogeneous mixture A glass of lemonade (mixture of water, lemon juice, sugar, salt) is a homogeneous mixture because the dissolved sugar, salt, and lemon juice are evenly distributed throughout the entire sample You can't easily separate the lemon juice from the water;




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Heterogeneous And Homogeneous Mixture Differences Videos Examples
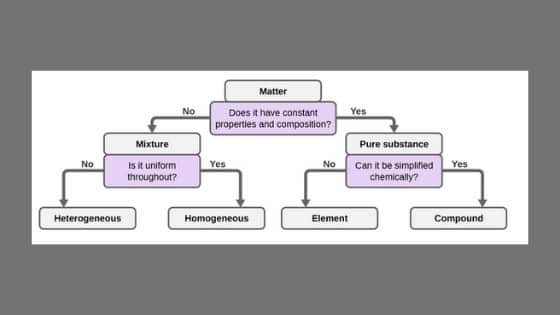
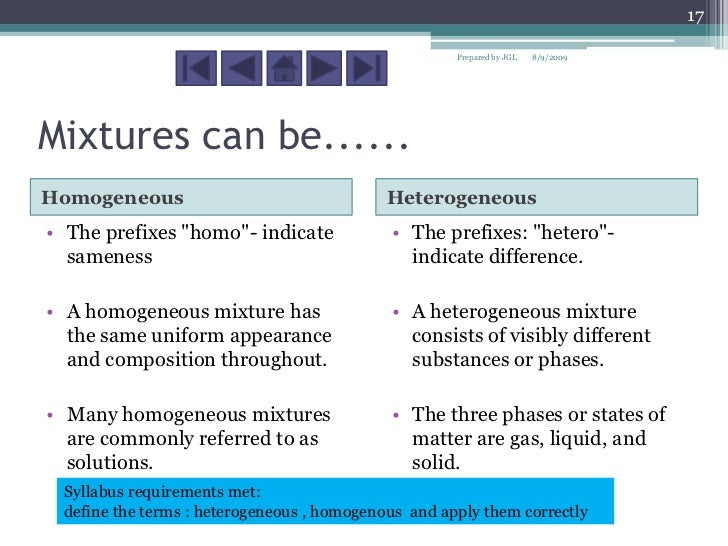
Heterogeneous mixtures definition, characteristics and examples by psychologysays Although they can be classified in several ways as a rule, we can find them homogeneous mixtures or heterogeneous mixtures, being on the latter of which we will speak throughout this article Homogeneous mixture Heterogeneous mixture It has a uniform composition It has a nonuniform composition It has only one phase There are two or more phases It can't be separated out physically It can be separated out physically 'homo' means the same 'hetero' means different Example a mixture of alcohol and waterMixtures involve combining pure compounds and possibly elements according to the heterogeneous definition and homogeneous definition as described above Exercise 1 Describe the following as an element, a compound, a homogenous mixture, or a heterogeneous mixture




Homogenous And Heterogenous Mixtures Chemistry For Non Majors



Mixture
In physical chemistry and materials science, the definition of a heterogeneous mixture is somewhat different Here, a homogeneous mixture is one in which all components are in a single phase, while a heterogeneous mixture contains components in different phases Examples of Heterogeneous MixturesBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is Definition of Heterogeneous Mixtures A mixture is a combination of two or more pure substances in which the original substances retain their chemical properties In some mixtures, the initial




Warm Up Define These Terms Mixtures Elements Compounds Heterogeneous Ppt Video Online Download



Homogeneous Mixture And Heterogeneous Mixture Ncert Books
This means that the different components of the mixture cannot be distinguished from one another in some wayHomogeneous Mixtures Heterogeneous Mixtures Particles are uniformly distributed throughout the mass Particles are nonuniform throughout the mass Homogeneous mixtures have the same composition throughout the mixture Heterogeneous mixtures have different compositions throughout the mixture The particles cannot be separated physicallyThe difference between homogeneous mixtures and heterogeneous mixtures is a matter of scale the heterogeneous mixture can be seen on beaches where sand included many particles like coral, shells and organic matter, etc they all can be separated easily hence known as a heterogeneous mixture but when we take a large amount of sand, it's impossible to separate all the matter,




Homogeneous Mixture Definition Examples Tutors Com




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
Can homogeneous mixtures be separated into their components?Anishkprateek anishkprateek Biology Secondary School Define homogeneous and heterogeneous mixture Homogeneous Mixture isn't really visible to the human eye but can be seen under a magnification lens Whereas heterogeneous mixtures can be seen with the human eye and under a magnification lens Känd som Homogeneous Mixtures are commonly known as solutions (since the solute and solvent are mixed thoroughly)




Homogenous And Heterogenous Mixtures Chemistry For Non Majors



Mixtures And Their Separations
Learn the definition of 'homogeneous and heterogeneous mixtures' Check out the pronunciation, synonyms and grammar Browse the use examples 'homogeneous and heterogeneous mixtures' in the great English corpusHeterogeneous mixture is a mixture with a nonuniform composition When you mix two components that remain separate from each other, that mixture is called a Heterogeneous mixture Examples of Heterogeneous mixture Concrete is an example of a Heterogenous mixture A mixture of Cement and Water A mixture of cold drinks and ice cube is also an example of a Heterogeneous mixture Homogeneous vs Heterogeneous Mixtures Mixtures can be either heterogeneous or homogeneous Unlike a heterogeneous mixture, a homogeneous mixture is a mixture that is uniform throughout;




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures



Homogeneous Mixture Experiment Qs Study




What Is A Homogeneous Mixture Definition And Examples




Compound Vs Mixture Difference And Comparison Diffen



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com




What Is The Difference Between Homogeneous And Heterogeneous Mixture



Ncert Solutions Is Matter Around Us Pure Chemistry Class 9




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo
:max_bytes(150000):strip_icc()/definition-of-pure-substance-605566_FINAL-d1c54ff9183944028aa8e213936affdf.png)



Heterogeneous Vs Homogeneous Mixtures



Homogeneous And Heterogeneous Mixtures



1




Homogeneity And Heterogeneity Wikipedia




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets



Mixture




What Is The Difference Between Homogeneity And Heterogeneity




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube



3




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




A Z Of Classification Of Matter What Is The Classification Of Matter By Science Books Medium



Difference Between Homogeneous Heterogeneous Mixtures Biomadam



Heterogeneous And Homogeneous Mixture Differences Videos Examples




Unit 3 Pure Substances And Mixtures Ppt Video Online Download




Homogeneous Mixture Definition




10 Homogeneous Mixture Examples In Daily Life Studiousguy




Is Salty Water Homogeneous Or Heterogeneous




Examples Of Homogeneous Mixtures Solid Liquid And Gas




Homogenous Definition And Examples Biology Online Dictionary




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com



Homogeneous And Heterogeneous Mixture Nine Science




How To Identify Heterogeneous Homogeneous Mixtures




Matter Mixtures Pure Substances Elements Compounds Heterogenous Ppt Video Online Download




Heterogeneous Vs Homogeneous Definitions The Prefix Homo Indicate Sameness Homogeneous A Homogeneous Mixture Has The Same Uniform Appearance Throughout Ppt Download



Homogeneous Mixtures Examples Definition And Types




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture Bitwise Academy



Is There Any Difference Between Homogeneous Mixture And Solution Here On Quora Previous Answers Are Vague About This While All My Textbooks And Google Sites Say They Are Exactly Same Quora



Homogeneous Mixture




Heterogeneous Mixture And Homogeneous Mixture Youtube
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Q2 Differentiate Between Homog Lido




2 2 Mixtures Classification Of Matter Siyavula



Q Tbn And9gctjuuyeomalljsdlz1u 5uxw8ihdx0o5wibwkoouvfqqtwvrcm3 Usqp Cau




Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Youtube




Homogeneous Mixture Definition Examples Tutors Com




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




Homogeneous Vs Heterogeneous Mixtures Difference And Comparison Diffen




10 Examples Of Mixtures




Homogenous Definition And Examples Biology Online Dictionary




Is Chocolate Chip Cookie Dough Ice Cream Heterogeneous Or Homogeneous



Homogeneous Vs Heterogeneous Mixtures



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
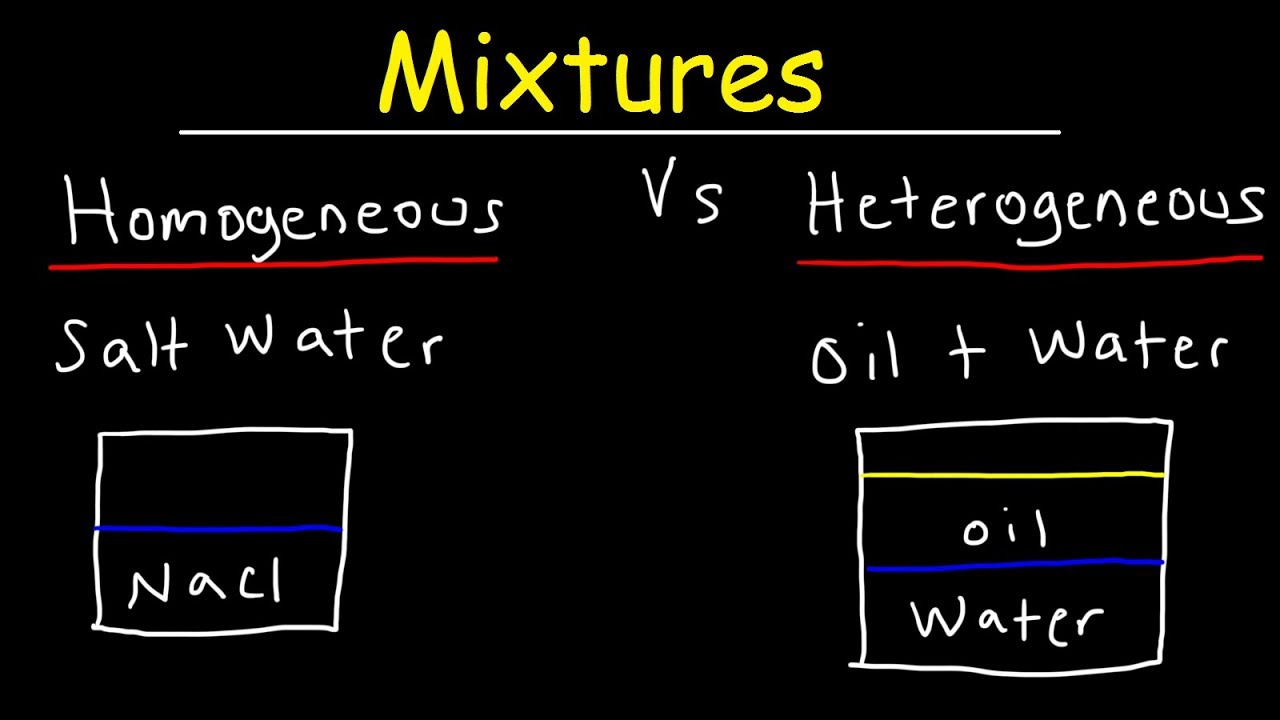



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube
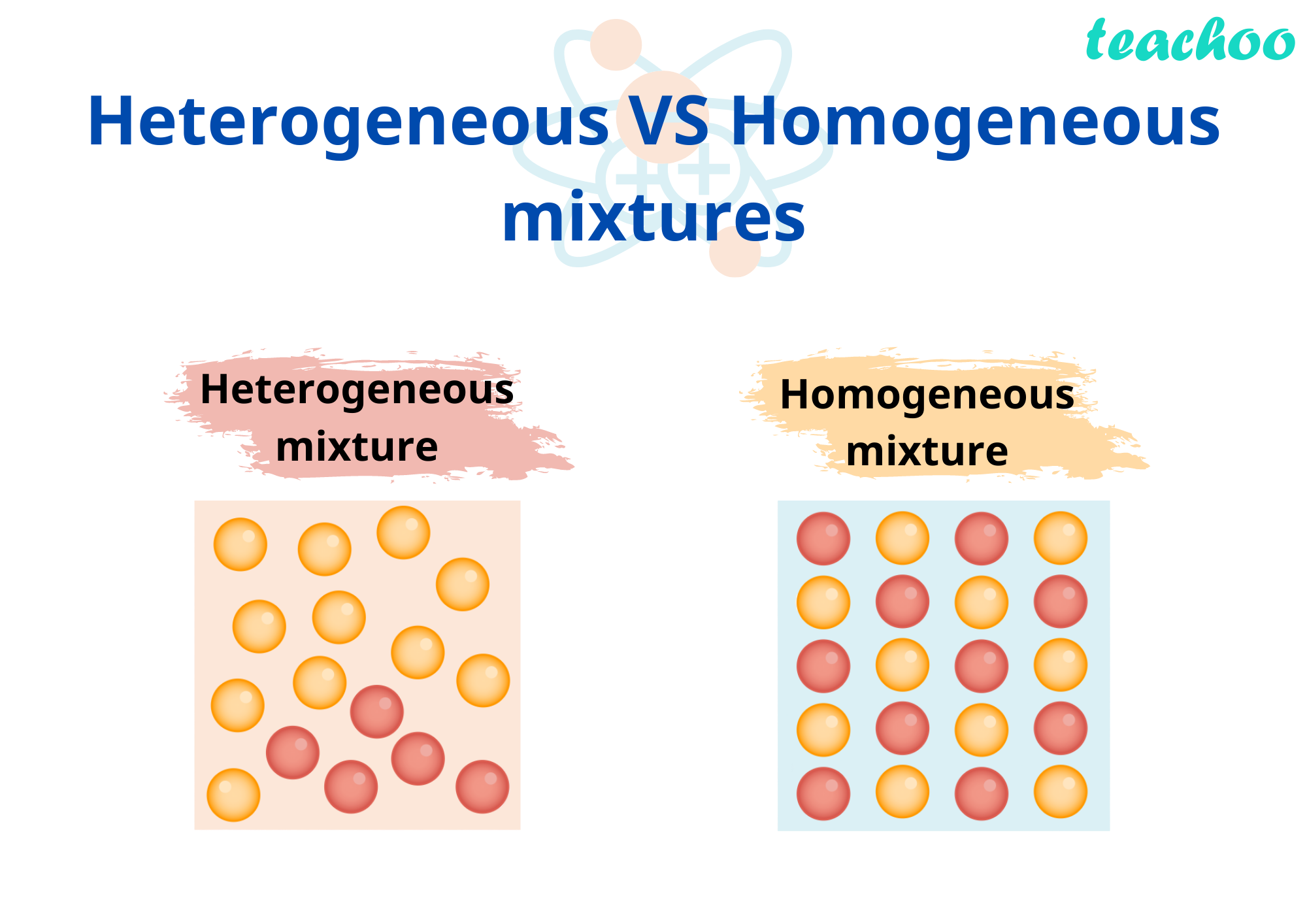



Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo
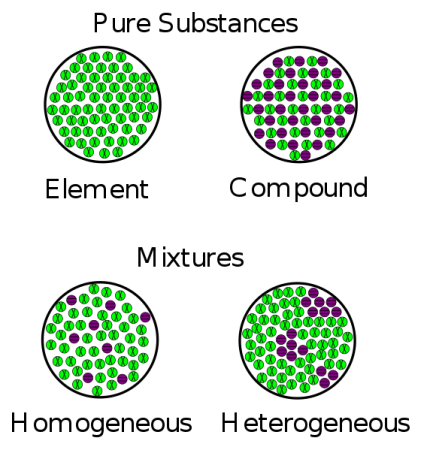



Heterogeneous Mixture Definition Science Trends




Chemistry For Kids Chemical Mixtures




Solutions Unit 3 Solution It Is A Homogeneous Mixture That Is Formed When A Substance Is Dissolved In Another Substance Ppt Download




Ch 12 1 Types Of Mixtures Ppt Video Online Download




Homogeneous Vs Heterogeneous Mixture Youtube



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Lesson Explainer Mixtures Nagwa




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous




Heterogeneous Vs Homogeneous Mixtures




Examples Of Heterogeneous Mixtures Types Made Simple




Introduction And What Is A Mixture Types Classification Video Examples
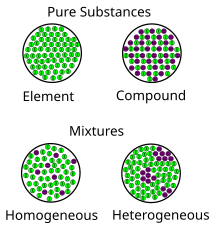



Mixture Wikipedia




Difference Between Homogeneous Mixture And Heterogeneous Mixture




How To Identify Heterogeneous Homogeneous Mixtures




Homogeneous Mixture Example Food




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




What Is A Heterogeneous Mixture Definition And Examples




Pin On Middle School Chemistry



1



Mixture


